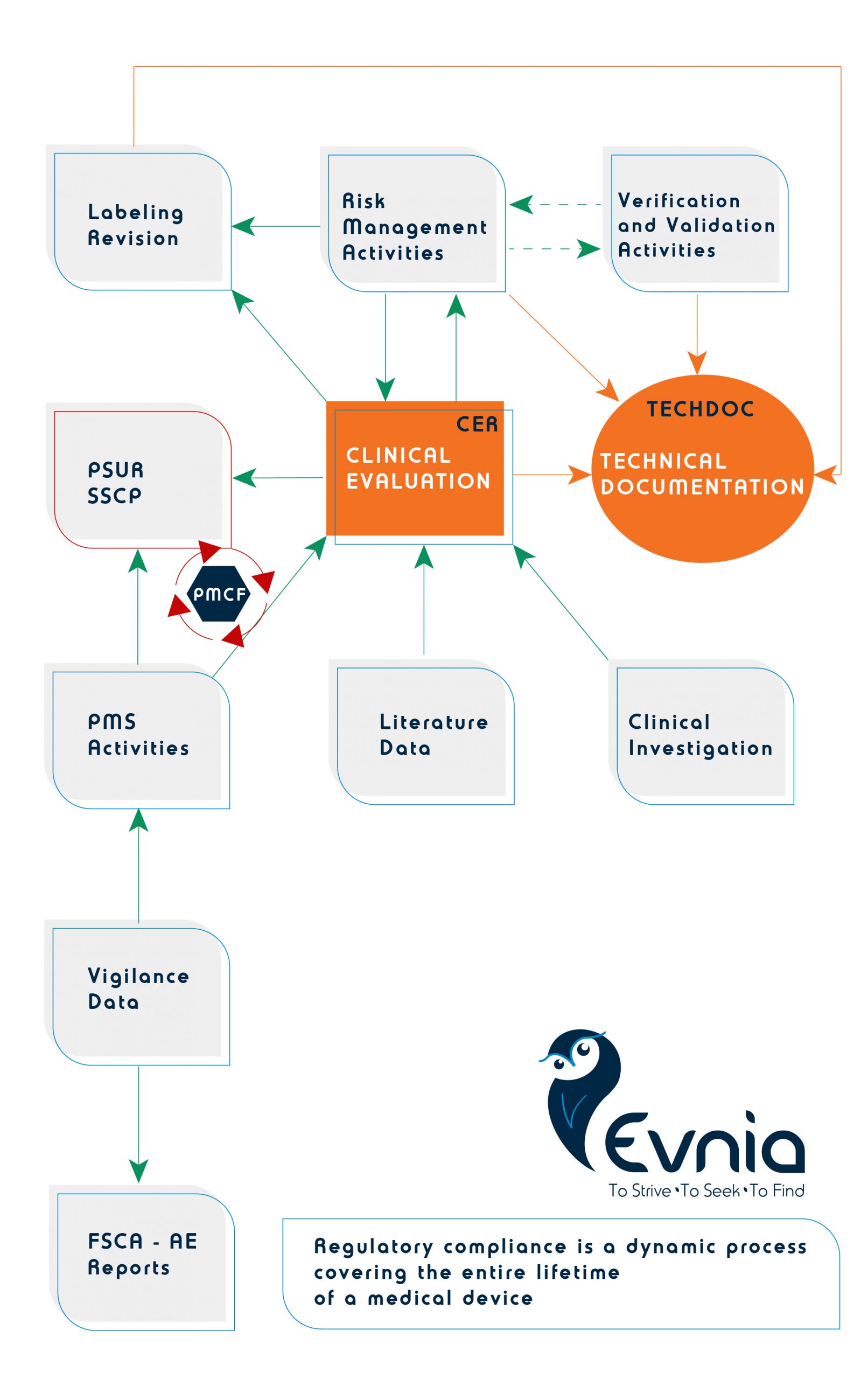
Do you ever wonder how your company’s regulatory compliance will fit into the new, increased EU-MDR requirements for safety, PMS data and clinical evidence?
Here’s a tip from Evnia: think (and treat) regulatory compliance as a dynamic process covering the entire lifetime of a medical device. There is no ‘’partial’’ compliance, and there is no one-way interaction. Get your QMS updated, align your risk management activities with literature data and V&V, define your PMCF needs, revise your labeling if available evidence says you need to and build your TechDoc in a ‘’clear, organised, readily searchable and unambiguous manner’’.
Need help with that? Contact Evnia today and change your perspective! EU-MDR is an opportunity for growth when you work with a knowledgeable and experienced partner.