At Evnia, we are commited to the management of complicated, international regulatory projects with a proactive mindset.
In her latest article in MedTech Outollok Europe, Evnia’s Regulatory Compliance Lead, Dr. Vicky Valla, explains our modus operandi, priorities and goals. Staying away from bureaucratic loops, Evnia uses Regulations to support manufacturers of all sizes in their efforts to accelerate markt access. To do so, we are deploying an holistic approach that translates regulatory needs (and gaps) into tangible, cost-effective, strategies.
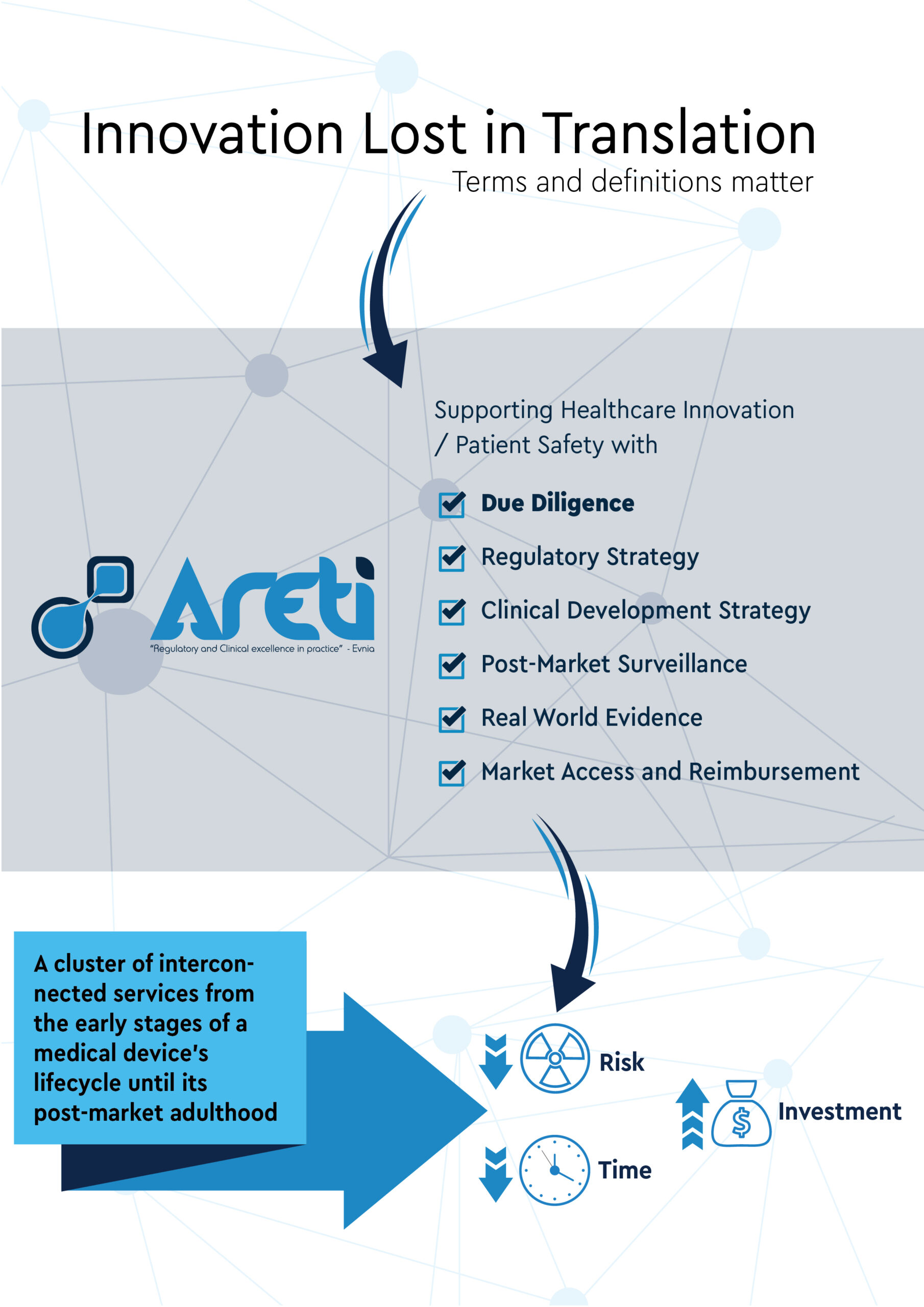
Evnia’s RWE Department uplifts our cluster of provided services by enhancing our capacity to monitor and evaluate medical devices and in-vitro diagnostics throughout their lifecycle. This is how we, practically, support healthcare innovation and patient safety: we help manufacturers developan investement-friendly, compliant portfolio!
Read more here: Evnia’s journey to the Holy Land of data integrity and patient safety