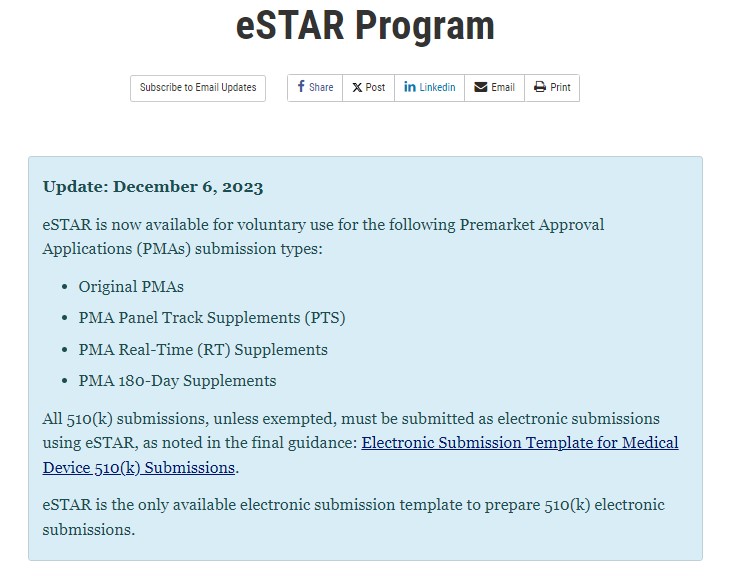
On December 6, 2023, the FDA evised its eSTAR templates (we are now in version 5.0) for in-vitro diagnostics and non-in vitro diagnostics also adding the option for the submission of original PMAs.
✔ PMA Panel Track Supplements (PTS)
✔ MA Real-Time (RT) Supplements
✔ PMA 180-Day Supplements
➡ 𝐍𝐨𝐭𝐞𝐬: Modular PMAs are currently not encompassed within the scope of eSTAR. Use of eSTAR for PMA submissions remains voluntary until the final guidance is published and there will be a transitional period.
📌 𝒆𝑺𝑻𝑨𝑹 𝒗𝒆𝒓𝒔𝒊𝒐𝒏 5 becomes a mandatory template for #510k submissions as of February 4th, 2024.
Read more on FDA’s site here
The new updates and set of changes are summarized in our presentation! Download it here eSTAR
Reach out if you are looking for a team of FDA experts who will support you throughout your premarket notification process!