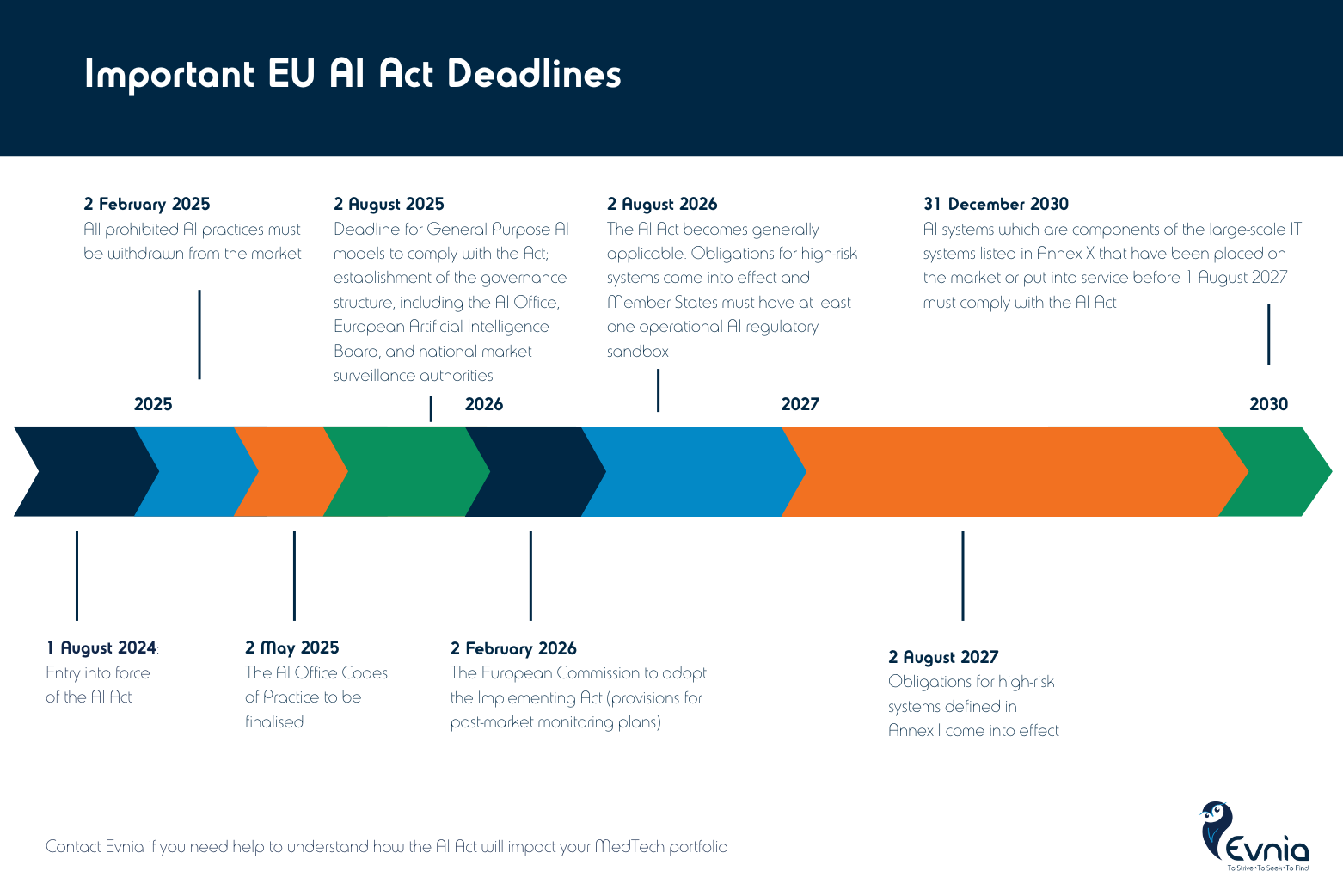
On August 1, 2024, the EU AI Act officially entered into force, marking a major milestone in AI regulation.
The staggered compliance deadlines will come into force over the course of three years, as presented in this video.
📌 AI systems used in medical devices will be within the scope of the AI Act due to MDR’s classification Rule 11. When requirements of the AI Act and MDR overlap, the manufacturer will be able to integrate the necessary measures to comply with the AI Act into the MDR procedures. The AI Act allows a single conformity assessment under the MDR (single technical documentation, declaration of conformity, and CE mark).
📌Medtech companies may need to satisfy the AI Act’s requirements both as “providers”, i.e., manufacturers or developers of AI systems, and “deployers” of AI systems depending on the AI system’s utilization. The rules for deployers focus on using AI systems by their intended purpose. Providers of in-scope devices will be subject to both the AI Act and the MDR or IVDR.
📌New requirements set by the AI Act are related to #datagovernance, automatically generated logs, human oversights, data transparency and provision of information to deployers, and accessibility requirements.
📌When is a medical device considered high-risk under the AI Act?
Used as a safety component of a product OR the AI system itself is a product + device covered under the Union Harmonisation Law (Annex I) + Device subject to 3rd party assessment under the Union Harmonisation Law.
Contact Evnia if you need help to understand how the AI Act will impact your Medtech Biotech Pharma portfolio!